
Water’s dipole moment is depicted in Figure. The structure of H2O is bent (by VSEPR theory) because of the lone pair on oxygen, which means that the vectors encoding the dipole moment of each bond do not cancel each other out. It uses the idea of electric dipole moment, which is a measure of how far negative and positive charges are separated in a system. The bond dipole moment determines the polarity of a chemical bond between two atoms in a molecule. The difference in electronegativity between two chemically linked atoms causes dipole moments. As a result, they can be found in both ionic and covalent bonds. When there is a charge separation in a system, a dipole moment occurs. The dipole moment is measured in Debye units, which are equal to the charge multiplied by the distance between the charges (1 Debye = 3.34×10-30cm). The dipole moment (μ) is used to calculate the dipole’s size. What is a dipole moment?Īn electric dipole is formed when two electrical charges of opposing sign and equal magnitude are separated by a distance.

Oxygen has a partial negative charge, while each hydrogen has a partial positive charge due to differences in electronegativity and lone electrons. The water molecule, which consists of one oxygen atom and two hydrogen atoms, is one of the most prevalent instances.
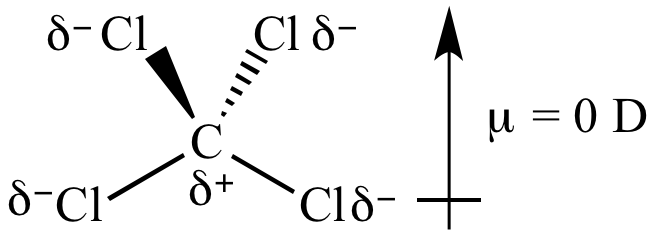
This happens when one atom is more electronegative than another, causing that atom to pull harder on the shared pair of electrons. When electrons are shared unequally among atoms in a molecule, a dipole moment is formed.


 0 kommentar(er)
0 kommentar(er)
